Acid Rain
Acid rain results when sulfur dioxide (SO2) and nitrogen oxides (NOX) are emitted into the atmosphere and transported by wind and air currents. The SO2 and NOX react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. The major sources of SO2 and NOX in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two thirds of SO2 and one fourth of NOX in the atmosphere come from electric power generators.
- Vehicles and heavy equipment.
- Manufacturing, oil refineries and other industries.
Winds can blow SO2 and NOX over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources.1
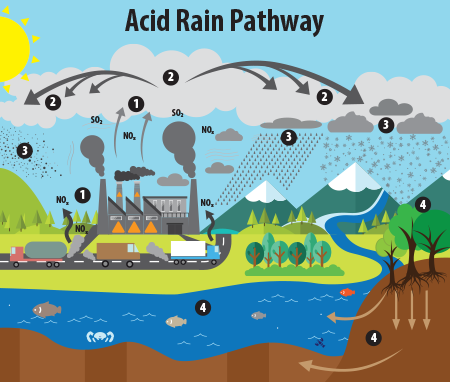
This image illustrates the pathway for acid rain in our environment: (1) Emissions of SO2 and NOx are released into the air, where (2) the pollutants are transformed into acid particles that may be transported long distances. (3) These acid particles then fall to the earth as wet and dry deposition (dust, rain, snow, etc.) and (4) may cause harmful effects on soil, forests, streams, and lakes.
Information and images on acid rain are courtesy of the United States Environmental Protection Agency .